Recovery Act enhances human subjects research
October 2010 | Volume 9, Issue 5
Research involving human subjects got a boost from the Recovery Act with $650,000 in one-year grants awarded to 12 projects. The Program to Enhance NIH-Supported Global Health Research Involving Human Subjects is providing support for U.S. investigators who work with developing country counterparts on sustainable electronic systems, communications, scientific and ethical competencies and ethics training.
To strengthen protection for human research subjects in Papua New Guinea, researchers will study regulatory barriers, protocol reviews and compliance monitoring. In the Republic of Georgia, an online platform will be piloted to enhance joint reviews of research practices by institutional review boards (IRBs) in the U.S. and overseas. Their materials and tools will be adaptable for training needs in other cultures and settings.
The funding will enable the Kenya Medical Research Institute to enhance its ethics review capacity required to deal with an increase in the volume and complexity of its projects. It will establish an international IRB that joins Indiana University and Moi University School of Medicine in Kenya.
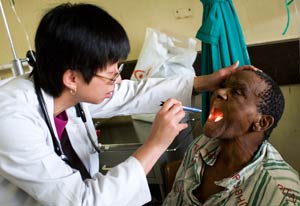
Photo by David Rochkind
Training to enhance clinical research involving human
subjects in the developing world is being supported
with Recovery Act funding.
The Botswana Research Ethics Network intends to use its funding to find ways to open channels of communication about ethical, procedural and cultural challenges between review board representatives in Africa and the U.S.
Other human subjects research enhancements will be supported in Colombia, China, India, Nigeria, Pakistan, South Africa and Tanzania.
More Information
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.