Microbicides provide new hope for HIV prevention in women
March / April 2012 | Volume 11, Issue 2

Photo by Jeff Gray
Fogarty grantee Dr.
Quarraisha Abdool Karim
recently presented her
microbicide research to
NIH staff.
Young women bear the brunt of the HIV epidemic in sub-Saharan Africa, acquiring the virus much earlier than men, and having access to fewer options for prevention.
That's why longtime Fogarty grantee Dr. Quarraisha Abdool Karim said she's been searching for a microbicide gel that females can control and that would be effective at preventing heterosexual transmission. As associate director of the Centre for the AIDS Programme of Research in South Africa, or CAPRISA, she knows the staggering statistics all too well.
"The difference between genders is a key driver of the epidemic," she observed during a recent address to NIH staff. "There is no end in sight," she said, with large numbers of African women continuing to become infected while still in their teens.
In a study of HIV prevalence in a rural area of South Africa, by age 14, only about 1 percent of boys were infected while 2.2 percent of girls were, she noted. By the age of 19 to 20, the boys' infection rate had only risen to 1.1 percent while the girls' rate had leapt to 16 percent. "We simply cannot allow this to continue," she stressed.
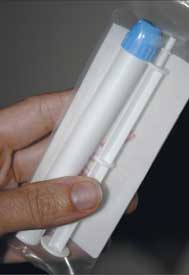
Photo by Louise Borst
The CAPRISA 004 trial
demonstrated for the first
time that 1 percent tenofovir
gel, pictured here, was
efficacious in preventing infection.
Karim - who also holds faculty positions at the University of KwaZulu-Natal in Durban and Columbia University in New York - reported her team has also studied HIV infection among sex workers, since they play a key role in the disease's spread. The researchers discovered the women earn 25 percent less for sex if they insist their clients wear a condom. That provided the impetus for the microbicide trial in this population.
Over the past two decades, substantial effort has been directed toward funding a microbicide that would work. Karim acknowledged the role the NIH and other funders played in supporting this critical work. Since 1998, Fogarty has supported research training for more than 400 African scientists through CAPRISA's partnership with Columbia, she noted. In addition, the National Institute of Allergy and Infectious Diseases has provided substantial research funding since 2002.
Now the hard work is finally paying off. In July 2010, the CAPRISA 004 trial demonstrated for the first time that 1 percent tenofovir gel was efficacious in preventing infection. Lauded as "Breakthrough of the Year" by Science magazine, the results encouraged policymakers to begin to have hope the epidemic could be controlled. Further analysis of the data has identified several key lessons for enhancing the efficacy of tenofovir gel and informing the conduct of future trials. These include the difficulty in achieving adequate adherence, the importance of ensuring there are high vaginal concentrations at the time of exposure and better understanding the increased risk of infection if genital inflammation is present.
If all goes well, she and her team will have regulatory approval and be able to launch the gel by the end of 2016. That gives her great hope, she said. Karim predicted successful implementation of the microbicide could prevent about 1.3 million new infections in South Africa alone.
More Information
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.