MenAfriVac vaccine slashes meningitis cases in Africa
May / June 2016 | Volume 15, Issue 3

Courtesy of PATH/Gabe Bienczycki
The MenAfriVac vaccine has nearly eliminated meningitis A in 26
countries in Africa since its 2010 rollout.
By Karin Zeitvogel
It's been hailed as a public health breakthrough and one of the biggest immunization success stories in Africa: a globally developed vaccine against meningitis A that has all but eliminated the deadly disease from the continent since its rollout in 2010.
"We have achieved something truly historic with MenAfriVac (TM) - creating an affordable, effective, tailor-made vaccine for Africa," said Steve Davis, president and CEO of the global health nonprofit PATH, which in 2001 partnered with the WHO to set up the Meningitis Vaccine Project (MVP).
With a 10-year, $70 million grant from the Bill & Melinda Gates Foundation, MVP set out to develop an affordable vaccine against meningitis A, a bacterial infection that attacks the lining of the brain and spinal cord and kills around 10 percent of those who are infected. In Africa's "meningitis belt" - which stretches from Senegal in the west to Ethiopia in the east - tens of thousands of people died in meningitis epidemics that swept across the continent every seven to 14 years. Most of the victims of the crippling and deadly illness were children and people under the age of 30.
PATH and WHO have hailed the international effort that led to MenAfriVac being developed "in record time and at less than one-tenth the cost of a typical new vaccine." NIH helped to transfer the conjugation technology used in the vaccine, developed by the FDA, to the Serum Institute of India at almost no cost. Serum and SyncoBioPartners of the Netherlands provided the raw materials for the conjugate vaccine, and Serum produced it for less than 50 cents per dose.
That price point is key to MenAfriVac's success. An African health official told MVP in 2001, "Please don't give us a vaccine that we can't afford. That's worse than no vaccine."
MenAfriVac was given to 20 million people in Burkina Faso, Mali and Niger when it was rolled out in 2010. By the end of that year's peak meningitis period - which falls during the dry season, roughly the northern hemisphere winter and spring - the three countries reported no cases of the deadly disease among those who received MenAfriVac.
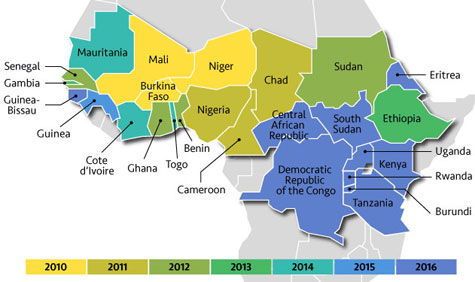
Reproduced with permission from www.path.org, 4/29/2016
Since 2010, MenAfriVac has been rolled out in Africa’s meningitis belt. For full details see long description below.
Since that first round of vaccinations, 235 million people in Africa's meningitis belt have received MenAfriVac. Cases of meningitis A have fallen from over 250,000 during an outbreak in 1996 to just 80 confirmed cases in 2015, according to scientists at the final conference of MVP, held in February 2016. Ninety percent of people who were vaccinated with MenAfriVac in 2010 had protective antibodies in their system five years later, the WHO has said.
But scientists and advocates have warned of "catastrophic resurgences in disease" if MenAfriVac vaccination campaigns do not continue.
"Our dramatic gains against meningitis A through mass vaccination campaigns will be jeopardized unless countries maintain a high level of protection by incorporating the meningitis A vaccine into their routine childhood immunization schedules," said Dr. Jean-Marie Okwo-Bele, WHO's director of Immunization, Vaccines and Biologicals, in November 2015. Since then, eight meningitis belt countries have applied for funding to allow them to give MenAfriVac to children and infants.
Following the success of MenAfriVac, the Serum Institute is partnering with PATH to produce another affordable vaccine, targeting meningitis A, C, W, X and Y. Clinical trials of the pentavalent vaccine are expected to begin this year.
More Information
Long description of map of countries in Africa's meningitis belt colored to indicate the year MenAfriVac was rolled out:
2010 - Burkina Faso, Mali, Niger
2011 - Cameroon, Chad, Nigeria
2012 - Benin, Ghana, Senegal, Sudan
2013 - Ethiopia, Gambia
2014 - Cote d'Ivoire, Mauritania, Togo
2015 - Guinea
2016 - Burundi, Central African Republic, Democratic Republic of Congo, Eritrea, Guinea Bissau, Kenya, Rwanda, South Sudan, Tanzania, Uganda
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.