NIAID begins human trials of universal flu vaccine candidate
May / June 2019 | Volume 18, Number 3
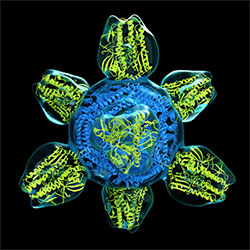
Image courtesy NIAID
Prototype for a universal flu vaccine.
The first clinical trial of an innovative universal influenza vaccine candidate began this spring and will examine the vaccine’s safety and tolerability as well as its ability to induce an immune response. Scientists at the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) developed the experimental vaccine, known as H1ssF_3928.
The vaccine candidate is designed to teach the body to make protective immune responses against diverse influenza subtypes by focusing the immune system on a portion of the virus that varies relatively little from strain to strain. The vaccine candidate was developed as part of a broader research agenda to create a so-called “universal” influenza vaccine that can provide long-lasting protection for all age groups from multiple influenza subtypes, including those that might cause a pandemic.
Annual flu epidemics worldwide are estimated to sicken 3 to 5 million people each year, resulting in as many as 650,000 deaths, according to the WHO.
NIAID expects the clinical trial to complete enrollment by the end of 2019 and hopes to begin reporting results in early 2020. For more information about the trial, visit
ClinicalTrials.gov and search identifier NCT03814720.
More Information
-
Dose, Safety, Tolerability and Immunogenicity of an Influenza H1 Stabilized Stem Ferritin Vaccine, VRCFLUNPF099-00-VP, in Healthy Adults (NCT03814720)
ClinicalTrials.gov, January 24, 2019 -
NIH begins first-in-human trial of a universal influenza vaccine candidate
Investigational vaccine designed to provide broad, durable protection from flu
NIAID/NIH news, April 3, 2019 -
Seasonal influenza fact sheet
WHO, updated November 6, 2018
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.