HHS makes progress on Zika, yellow fever vaccines
September / October 2016 | Volume 15, Number 5
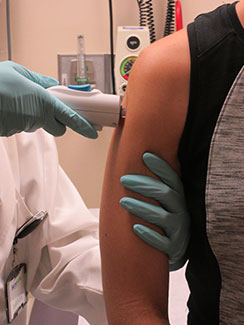
Photo courtesy of NIAID
A healthy volunteer receives the NIAID Zika
virus investigational DNA vaccine as part
of an early-stage trial to test the vaccine’s
safety and effectiveness.
The U.S. Department of Health and Human Services (HHS) has announced progress on several fronts to develop vaccines that protect against Zika and yellow fever viruses.
In early September, the Department announced it has awarded an $8.2 million contract to Moderna Therapeutics of Cambridge, Mass., to help speed development of a novel vaccine for Zika. Moderna’s vaccine candidate uses messenger RNA or mRNA technology. Messenger RNA is a molecule that carries specific genetic codes to parts of the cell. This type of vaccine uses mRNA containing the genetic sequence of the Zika virus to generate an immune response in people.
Meanwhile at NIH, a clinical trial was launched in August to evaluate the safety and efficacy of another investigational Zika vaccine developed by the National Institute of Allergy and Infectious Diseases (NIAID). At least 80 healthy volunteers are expected to be studied at three U.S. sites.
“A safe and effective vaccine to prevent Zika virus infection and the devastating birth defects it causes is a public health imperative,” said NIAID Director Dr. Anthony S. Fauci. “NIAID worked expeditiously to ready a vaccine candidate, and results in animal testing have been very encouraging.”
In addition, NIH researchers have identified compounds that potentially can be used to inhibit Zika replication and reduce its ability to kill brain cells. Using screening robots at the National Center for Advancing Translational Sciences (NCATS), researchers identified two classes of compounds effective against Zika. One is emricasan, an investigational drug being evaluated for liver ailments. The other is niclosamide, an approved drug to treat people who have worm infections.
Finally, NIAID has begun early-stage clinical study of a vaccine for yellow fever. The disease has recently caused more than 400 deaths and 5,000 infections in Angola and the Democratic Republic of the Congo.
More Information
To view Adobe PDF files,
download current, free accessible plug-ins from Adobe's website.